Is Boiling Exothermic Or Endothermic
x.03: Energy in Chemical and Physical Changes
- Page ID
- 178167
Skills to Develop
- Ascertain endothermic and exothermic reactions.
- Describe how heat is transferred in endothermic and exothermic reactions.
- Determine whether a reaction is endothermic or exothermic through observations, temperature changes, or an free energy diagram.
Then far we've talked nigh how energy exists equally either kinetic free energy or potential free energy and how energy tin be transferred every bit either estrus or work. While information technology'due south of import to empathize the difference between kinetic energy and potential free energy and the departure between oestrus and work, the truth is, free energy is constantly changing. Kinetic energy is constantly beingness turned into potential energy, and potential energy is constantly existence turned into kinetic energy. As well, energy that is transferred as piece of work might later end upwards transferred as heat, while energy that is transferred equally heat might later end up being used to do piece of work.
Even though free energy tin can change form, it must still follow one fundamental constabulary – Energy cannot be created or destroyed, it can only exist changed from 1 class to some other. This law is known as the Law of Conservation of Energy. In a lot of ways, energy is like money. You can exchange quarters for dollar bills and dollar bills for quarters, merely no matter how often y'all convert betwixt the two, you will not end up with whatsoever more than or any less coin than you started with. Similarly, y'all tin can transfer (or spend) money using cash, or transfer coin using a credit card, but you even so spend the same amount of money, and the store notwithstanding makes the same amount of money.
A campfire is an example of basic thermochemistry. The reaction is initiated past the application of oestrus from a match. The reaction converting forest to carbon dioxide and h2o (among other things) continues, releasing oestrus energy in the process. This heat energy can then be used to melt food, roast marshmallows, or simply keep warm when it's cold outside.

An paradigm of a campfire with colored flames, made by the burning of a garden hose in a copper pipage. Epitome used with permission (CC SA-BY 3.0; Jared)
Exothermic and Endothermic Processes
When physical or chemical changes occur, they are generally accompanied past a transfer of free energy. The police force of conservation of energy states that in whatsoever physical or chemical process, energy is neither created nor destroyed. In other words, the entire energy in the universe is conserved. In social club to better sympathize the energy changes taking place during a reaction, we demand to define two parts of the universe, chosen the organisation and the surround. The system is the specific portion of matter in a given space that is existence studied during an experiment or an observation. The surroundings is everything in the universe that is not role of the organization. In practical terms for a laboratory chemist, the system is the item chemicals being reacted, while the surroundings is the firsthand vicinity within the room. During most processes, energy is exchanged between the system and the surroundings. If the system loses a certain amount of energy, that same amount of energy is gained by the surround. If the organization gains a sure amount of energy, that energy is supplied by the environs.
A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. In the class of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. The quantity of heat for a process is represented past the alphabetic character \(q\). The sign of \(q\) for an endothermic process is positive because the system is gaining estrus. A chemical reaction or physical change is exothermic if rut is released by the organization into the surroundings. Considering the surroundings is gaining heat from the arrangement, the temperature of the environment increases. The sign of \(q\) for an exothermic process is negative considering the system is losing rut.
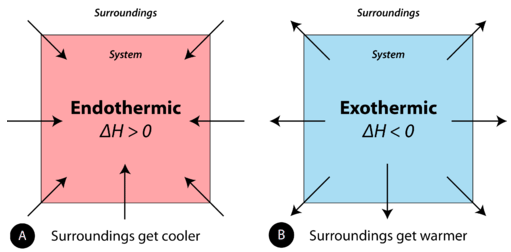
Effigy \(\PageIndex{1}\): (A) Endothermic reaction. (B) Exothermic reaction.
During phase changes, energy changes are usually involved. For case, when solid dry water ice vaporizes (physical change), carbon dioxide molecules blot energy. Meanwhile, when liquid water becomes ice energy is released. Recall that all chemical reactions involve a change in the bonds of the reactants. The bonds in the reactants are broken and the bonds of the products are formed. Chemical bonds have potential energy or "stored energy". Because we are changing the bonding, this ways we are also changing how much of this "stored free energy" there is in a reaction.
Energy changes are frequently shown by drawing an energy diagram. Free energy diagrams show the stored/subconscious energy of the reactants and products as well as the activation free energy. If, on an energy diagram, the products have more stored energy than the reactants started with, the reaction is endothermic. Y'all had to give the reaction energy. If, on the energy diagram, the products take less stored energy than the reactants started with, the reaction is exothermic.
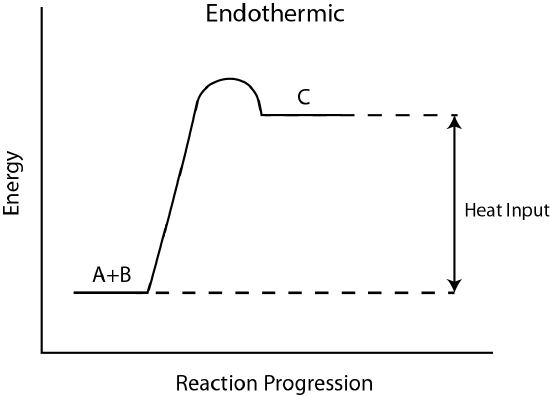
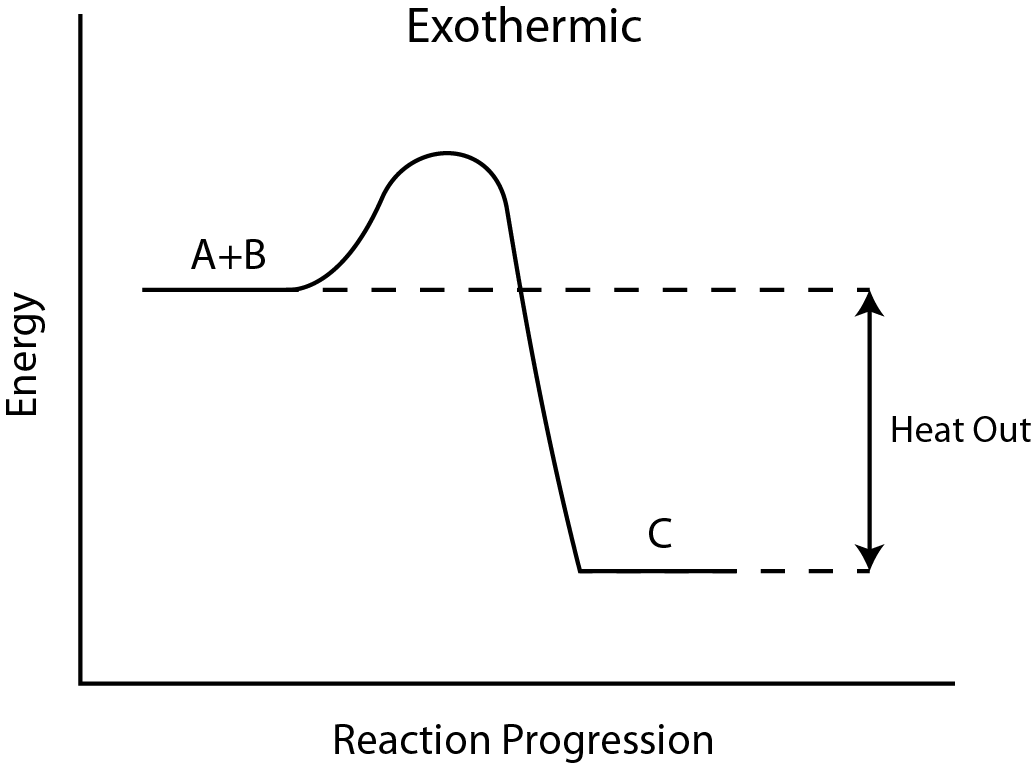
Figure \(\PageIndex{2}\): (A) Endothermic reaction Free energy Diagram. (B) Exothermic reaction Energy Diagram (By Brazosport Higher - Own work, CC Past-SA 3.0, https://commons.wikimedia.org/w/inde...curid=16113024)
Instance \(\PageIndex{ane}\)
Label each of the following processes as endothermic or exothermic.
- water boiling
- gasoline burning
- ice forming on a pond
Solution:
- endothermic - you must put a pan of h2o on the stove and give it heat in order to get water to eddy. Because you are adding heat/energy, the reaction is endothermic.
- exothermic - when yous fire something, it feels hot to you considering information technology is giving off estrus into the surroundings.
- exothermic - think of water ice forming in your freezer instead. You put water into the freezer, which takes heat out of the water, to get it to freeze. Considering estrus is beingness pulled out of the h2o, it is exothermic. Heat is leaving.
Exercise \(\PageIndex{i}\)
Characterization each of the following processes as endothermic or exothermic.
- water vapor condensing
- gold melting
- Answer (a)
- exothermic
- Answer (b)
- endothermic
Summary
Phase changes involve changes in free energy. All chemical reactions involve changes in energy. This may exist a change in heat, electricity, low-cal, or other forms of energy. Reactions that absorb free energy are endothermic. Reactions that release free energy are exothermic.
Is Boiling Exothermic Or Endothermic,
Source: https://chem.libretexts.org/Courses/Los_Angeles_Trade_Technical_College/Chem_51/10%3A_Introduction_to_Energy/10.03%3A_Energy_in_Chemical_and_Physical_Changes
Posted by: ernsttrive1971.blogspot.com


0 Response to "Is Boiling Exothermic Or Endothermic"
Post a Comment